Up-scaling
The demand for transfectable plasmid DNA in TGE approaches is high. Since only a small proportion of transfected plasmid DNA reaches the nucleus for efficient transcription, it appears necessary to have an excess of 105 – 108 plasmids per cell. Using a typical TGE plasmid, this corresponds to about 2 pg per cell and at least 10 mg per L at 5 x 106 cells per mL. To meet these requirements, bacterial systems (usually E. coli) are the most widely used hosts for the production of large amounts of plasmid DNA. However, bacterial components like endotoxins have cytotoxic effects on eukaryotic cells so a stringent preparation system is needed to produce high-quality plasmid DNA. Up to now, up-scaling has been limited by various scientific and economic bottlenecks regarding plasmid preparation. Commercially available plasmid purification kits exclusively utilize single-use columns and have a limited capacity of about 10 mg. In order to achieve a high-yielding plasmid preparation process, the manufacturing process of a TGE-optimized plasmid was compared in a matrix setup by using different E. coli strains cultivated in different media. Quality attributes were highly dependent on the host strains, whereas absolute yield was found to be a function of the cultivation medium. Additionally, a purification process was implemented using an anion exchanger with modified amino-ethyl groups for optimal separation of DNA isoforms. This resulted in 40 mg high-quality plasmid DNA per L culture volume on average. Up-scaling this process yields approx. 250 mg purified plasmid DNA per run.
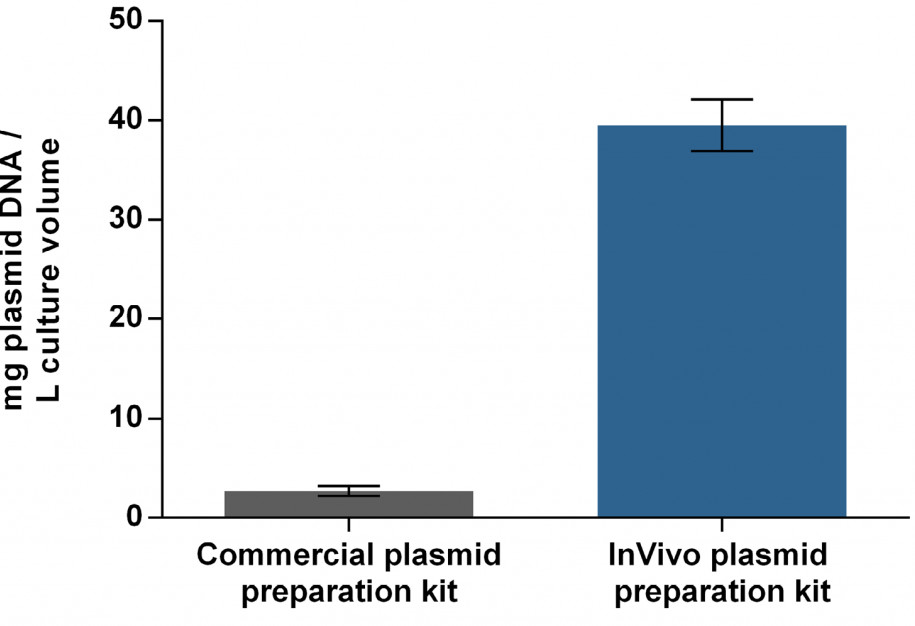
Plasmid yield for transient transfection using either InVivo’s plasmid preparation kit or a commercial plasmid preparation kit.